Easy functionalization of carbon nano-onions with disulfides and their use as recyclable heterogeneous organocatalysts
Abstract
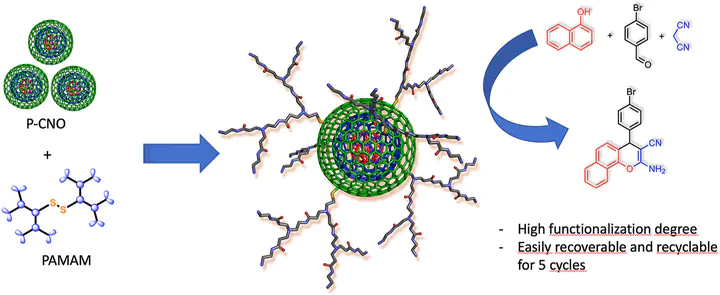
A facile functionalization of carbon nano-onions (CNO), through an atom economical one-step process, with a series of different generation (2.0, 2.5, and 3.0G) poly(amidoamine) (PAMAM) dendrimers with a cystamine core and endowed with either methyl ester or amino groups in the periphery has been reported. The radical addition reaction onto CNO surface, after the homolytic rupture of the S–S bond of cystamine, afforded CNO-PAMAM hybrids, which were extensively characterized using various analytical and spectroscopic techniques such as thermogravimetric analysis (TGA), attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy, solid state 13C NMR, Raman, X-ray photoelectron spectroscopy (XPS), dynamic light scattering (DLS) and zeta potential (ZP). Morphological and textural properties were also investigated by high-resolution transmission electron microscopy (HRTEM) analysis and N2 adsorption/desorption isotherms. Furthermore, the presence and availability of –NH2 groups has been demonstrated by applying the functionalized CNO with PAMAM 2.0G and 3.0G as heterogeneous and recyclable organocatalysts in the one-pot three-component synthesis of 2-amino-4H-chromene derivatives.